Testimonial: Car Accident
Ken shares his testimonial as a Shunnarah client after a car accident.
You trust your sleep apnea breathing device to help you at night. However, many patients using Philips CPAP and BiPAP devices have experienced serious medical complications. The company has recalled many devices due to health dangers. If you suffered significant injury after using one, you may be able to sue for compensation.
Case evaluations are 100% free and available 24/7
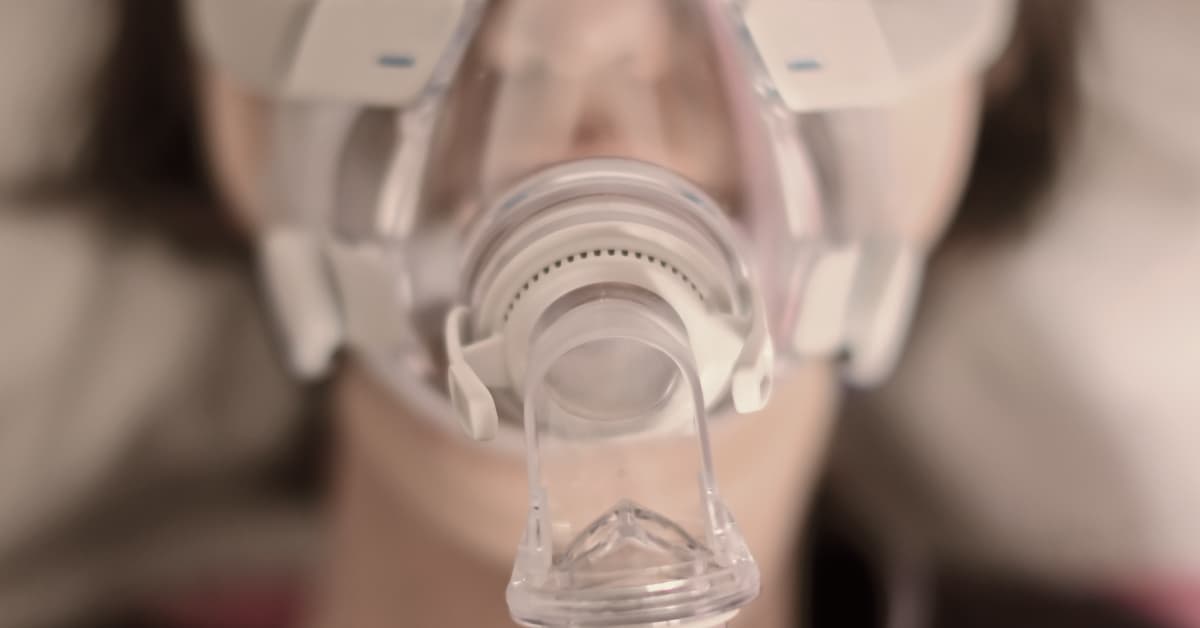
Philips CPAP (Continuous Positive Airway Pressure) and BiPAP (Bilevel Positive Airway Pressure) devices are innovative medical tools designed to provide crucial support for individuals suffering from sleep apnea and other related respiratory conditions. These devices play a vital role in ensuring that users maintain an unobstructed airflow throughout the night, leading to a more restful and healthier sleep pattern. Here’s a detailed look at their uses:
The Philips CPAP devices are engineered to deliver a steady stream of air through a mask and tubing system, creating a gentle pressure in the airways that keeps them open while the user sleeps. This continuous airflow prevents the pauses in breathing that are characteristic of sleep apnea, significantly reducing the risk of sudden awakenings and ensuring a deeper, more consistent sleep cycle. Ideal for those with obstructive sleep apnea, Philips CPAP devices are celebrated for their ability to improve sleep quality, enhance daytime alertness, and lower blood pressure in users.
While CPAP devices provide a constant level of air pressure, Philips BiPAP devices offer a more sophisticated approach, delivering two distinct levels of air pressure: one for inhalation (IPAP) and a lower one for exhalation (EPAP). This dual-pressure technology makes breathing more natural and comfortable for users, especially those who have difficulty tolerating the constant pressure from CPAP machines or those with more complex forms of sleep apnea. BiPAP devices are also beneficial for individuals with certain types of respiratory failure, providing critical support that can significantly improve breathing patterns and overall health.
On June 30, 2021, the FDA alerted the public that Philips recalled certain devices due to concerns regarding its polyester-based polyurethane sound abatement foam. (12) The FDA has recognized this as a Class I recall, “the most serious type of recall. Use of these devices may cause serious injuries or death.” (13)
“The off-gassed chemicals and foam particles may lead to serious or life-threatening injuries, difficulty breathing (respiratory distress), swelling (inflammation), a lack of oxygen (hypoxia), too much carbon dioxide (hypercarbia), or toxic reactions.” (14)
There have been 83 complaints reported as of July 22, 2021. All serial numbers of these types of devices and models have been recalled: (15) (16)
Continuous ventilator, minimum ventilatory support, facility use – E30.
Continuous ventilator, non-life supporting – DreamStation ASV; DreamStation ST, AVAPS; SystemOne ASV4; C-Series ASV; C-Series S/T and AVAPS; OmniLab Advanced+.
Noncontinuous ventilator – SystemOne (Q-Series); DreamStation; DreamStation Go; Dorma 400; Dorma 500; REMstar SE Auto.
Continuous ventilator – Trilogy 100; Trilogy 200; Garbin Plus, Aeris, LifeVent.
Continuous ventilator, minimum ventilatory support, facility use – A-Series BiPAP Hybrid A30 (not marketed in U.S.); A-Series BiPAP V30 Auto.
Continuous ventilator, non-life supporting – A-Series BiPAP A40; A-Series BiPAP A30.
According to the U.S. Food & Drug Administration (FDA), “The polyester-based polyurethane (PE-PUR) sound abatement foam, which is used to reduce sound and vibration in these affected devices, may break down and potentially enter the device’s air pathway. If this occurs, black debris from the foam or certain chemicals released into the device’s air pathway may be inhaled or swallowed by the person using the device.” (5)
On November 12, 2021, the FDA provided an update on the recall. The FDA had inspected a Philips facility “to determine what may have caused or contributed to the foam issues and to assess adherence to the agency’s quality system regulations.” (6)
During the inspection, the FDA learned that Philips’ replacement foam, which is silicone-based, may contain volatile organic compounds (VOCs). The FDA requested an independent laboratory test the foam for any potential safety risks.
Additionally, the FDA inspection revealed that Philips was aware no later than 2015 that the polyester-based polyurethane foam (PE-PUR) foam that is the subject of the recall had a degradation issue that was dangerous to users. Internal company testing also revealed the toxicity and potential carcinogenicity of the PE-PUR foam degradation products, yet Philips chose to keep using the PE-PUR foam over safer alternatives.
Philips Respironics, the manufacturer, used PE-PUR foam to lessen the sound and vibration of its breathing devices. However, if this foam comes into contact with your airways or digestive tract, dangerous side effects or medical complications may occur:
If the foam breaks down, you may inhale or swallow foam particles.
Foam particles may release toxic chemicals, which you can breathe in or ingest.
Inhaling or swallowing PE-PUR foam particles can lead to serious injury. Possible injuries can be life-threatening. Injuries may also be permanent. Medical treatment may be necessary to prevent irreversible damage.
Philips has received complaints from patients who have noted black debris, or particles, in their breathing device’s air pathway. Patients have reported headaches, irritation in their upper airway, chest pressure, a cough, and sinus infections. These side effects may be linked to inhalation of foam particles.
Long-term, permanent, and life-threatening health complications – including cancer – of PE-PUR foam are possible. If you are diagnosed with any of these conditions, your illness may be linked to your use of Philips CPAP and BiPAP:
If you or a loved one suffered serious medical complications or a health injury after using a recalled Philips medical device, you may be entitled to compensation. Compensation may cover medical expenses, as well as your pain and suffering, loss of earnings, and potential future income loss. Punitive damages may also be awarded.
Possible compensation you could receive includes:
Time is limited. If you are ready to file a claim for your medical complications or injury due to a recalled Philips medical device, call Shunnarah now.
Alexander Shunnarah Trial Attorneys handles cases in all 50 states. Find a location or attorney to assist you with your case.
Explore our FAQ to get the answers to some of our most frequently asked questions about Philips BiPAP and CPAP Devices
How do I know if my device is affected by the recall?
If you are using a Philips BiPAP or CPAP machine, it’s important to check the specific model and serial number against the list provided on the Philips website or through the FDA’s recall announcements. Philips has also recommended that users of recalled devices stop using them and contact their healthcare providers to discuss alternatives for their sleep apnea therapy.
What should I do if I believe I have been harmed by a Philips respiratory device?
If you have experienced adverse health effects that you believe are linked to using a recalled Philips CPAP or BiPAP device, you should first seek medical attention to document your health issues. Then, collect any information regarding your device, including the model, purchase details, and any medical records related to your use of the device. Contacting a personal injury attorney who specializes in medical device claims is also a crucial step. They can help you understand your rights and options for pursuing a claim against Philips.
What type of compensation can I seek if I’ve been injured by a Philips CPAP or BiPAP machine?
Individuals affected by faulty Philips CPAP or BiPAP machines may be eligible for compensation for medical bills, treatment costs for conditions caused by the device, lost wages, pain and suffering, and potentially other damages. If you have suffered significantly, compensation might also cover future medical expenses and loss of quality of life. The specific compensation will depend on the severity of your injuries and the impact on your overall health and daily living. A skilled attorney can negotiate or litigate to secure the best possible outcome for your circumstances.
Available 24/7 for immediate case evaluations post-accident or injury.
Connect with a dedicated attorney who will aggressively pursue your compensation through trial if needed.
Receive top-tier legal guidance and representation. We don’t give up until you receive the compensation you deserve.